Gas phase chemistry


Gas phase chemistry

Stereospecific synthesis of hemiacetals
<The only method>
We accomplished stereospecific synthesis of individual hemiacetals in gas phase under collision induced dissociation conditions.
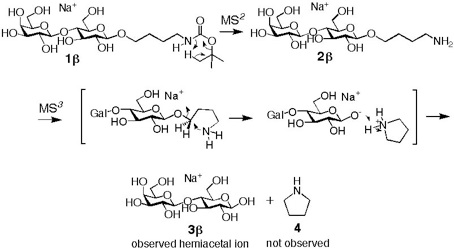
We first synthesized a Boc protected aminobutyl glycoside chemically. This compound was then subjected to mass spectrometric analysis where compounds were ionized as sodium adducts by electrospray ionization. The sodiated molecule was trapped in a quadrupole ion trap chamber and was subjected to a collision-induced dissociation conditions. The precursor ion then produced aminobutyl glycoside that lost the Boc protecting group. Interestingly here, we could observe that one of the proton was migrated from methyl group of tBu to a nitrogen atom when we used a molecule where all exchangeable protons were replaced by deuterions. The aminobutyl glycoside was once again isolated and further subjected to CID condition. This process produced sodiated hemiacetal species called C-ion.
We found that this reaction proceeded via five-membered transition state independent of anomeric oxygen. Thus, the resulting hemiacetal retains the initial anomeric configurations. This was the first time discovery of synthesizing hemiacetals stereospecifically.
These alpha and beta hemiacetals are very useful in analyzing anomeric configurations of naturally occurring oligosaccharide by comparing the C-ions obtained under CID conditions.