Kanie Laboratory welcome graduate students from abroad. Please contact for more infomation. About 1000 students from all over the world are studying at Tokai University.
We chemically synthesize carbohydrate-related molecules and use them in sensing elements against various chemical compounds. We also work on a project of miniature glycoprotein synthesis as a drug candidate.
東海大学工学部糖鎖工学研究室(蟹江研究室)では、糖化合物に注目して分子の化学センシング研究を進めています。有機化学と分析化学を身につけよう。
生物工学科
2023年3月19日 オープンキャンパスおこないました
夢ナビライブ参加中:2023年夏「研究室訪問」を行いました…高校生の皆さん!
学科のイメージ映像(YouTube)公開中です!
Research Interests:
Living things developed various sensory mechanisms against electromagnetic field, gravitation, chemical entities. sound, etc. for their survival. We are interested in developing artificial sensory system for chemical compounds especially focusing on surface chemical properties of individual molecules and analytical methods. The type of mechanism may provide a universal type of sensor interface to detect molecules, viruses, and microbes.
The followings are in Japanese. ===
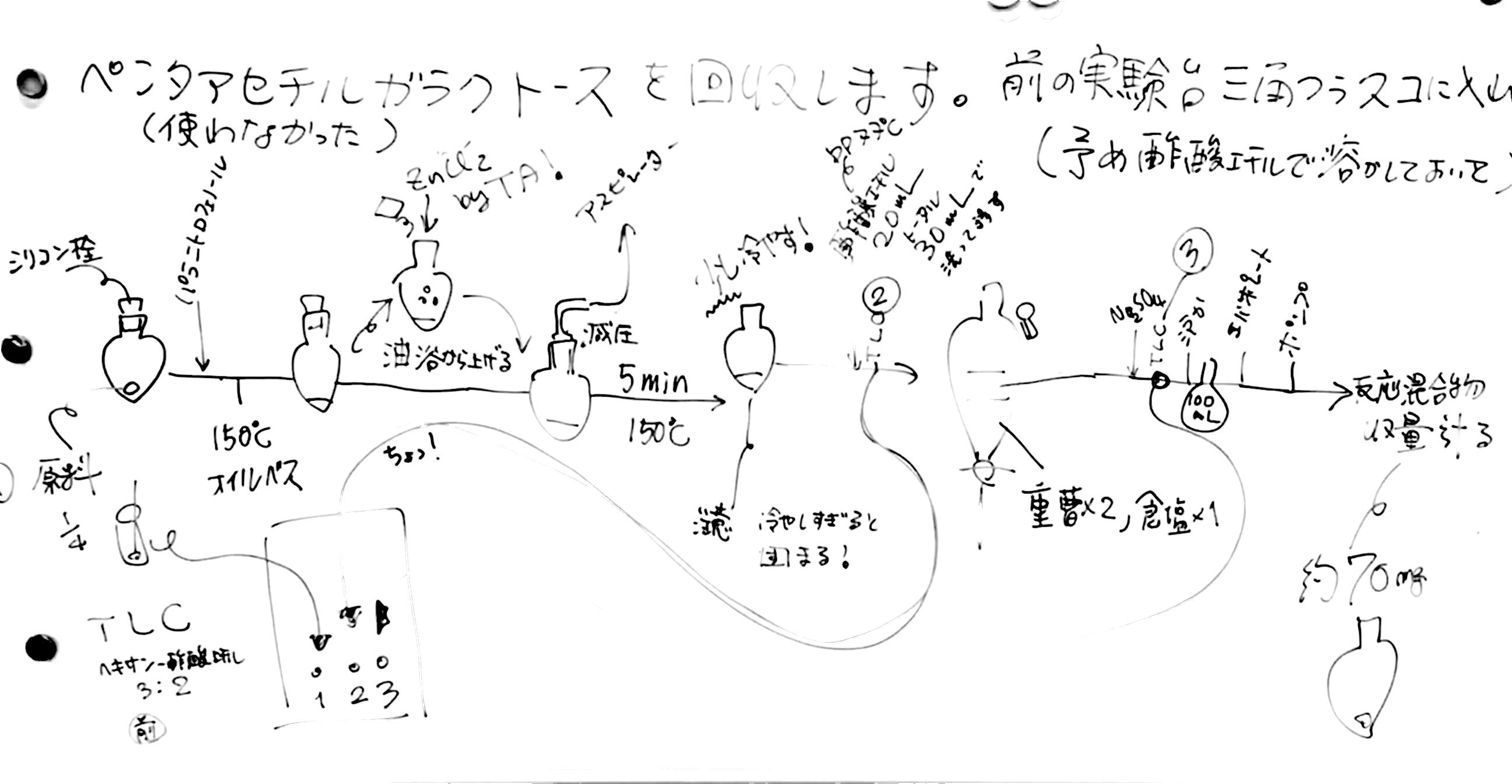
研究テーマ
私たち人類を含む生物は、様々な刺激に応答して生きています。そのような刺激には、電磁波、重力、化学物質、音波などを上げることができます。このような多様な刺激応答の中で、私たちの研究室では特に化学物質のセンシングに注目して研究を開始しています。
例えば、味覚や嗅覚は私たちの生活に非常に身近ですが、生物本来の機能としては、生存をかけ発達させた感覚であるという事ができます。また、このような感覚のメカニズムの初期段階は分子間の認識現象です。この認識反応は、一般的に低分子とタンパク質の間の特異的な結合によりますが、正確に表現すれば化合物を構成する官能基群の特定の空間配置とタンパク質の一部に形成されたアミノ酸側鎖、あるいは、骨格を形成するアミド結合によって形成された三次元空間内の官能基群の配置との間の親和性の総体であると表現できます。このような考えに基づき、私たちの研究室では官能基の組み合わせに注目するセンシング機構研究を行っています。
Regulatory mechanism of glycan synthesis inside cells…we used to work on…
Most of glycans are synthesized at ER and Golgi apparatus by cascade reactions of a series of glycosyltransferases utilizing sugar nucleotides, most of which are synthesized in cytosol and transported into Golgi rumen, however, the mechanism how such synthesis is controlled is not known. We address this issue by using synthetic probe molecule that carries reporter functionality. In particular, we utilize lactosyl ceramide (LacCer) analogs carrying BODIPY dyes. LacCer is located at the fork in a complex glycosphingolipid synthetic pathway. We combine chemistry and analytical chemistry to synthesize probe molecules and to analyze glycan transformation.
Due to a situation, we had to take a break on this subject, but we hope someone pursuit the type of research to understand the glycan processing mechanism in Golgi apparatus somewhere…
A list of our papers associated to this subject.
- Sequential enzymatic glycosyltransfer reactions on a microfluidic device: Synthesis of a glycosaminoglycan linkage region tetrasaccharide. Ono Y, Kitajima M, Daikoku S, Shiroya T, Nishihara S, Kanie Y, Suzuki K, Goto S, Kanie O.
Lab Chip. 2008 Dec;8(12):2168-73. doi: 10.1039/b809316d. IF 7.5
- Mechanism of a gas-phase dissociation reaction of 4-aminobutyl glycosides under CID MS/MS conditions. Shioiri Y, Suzuki K, Kanie O.
J Mass Spectrom. 2008 Aug;43(8):1132-9. doi: 10.1002/jms.1397. IF 2.4 - N-Hexyl-4-aminobutyl glycosides for investigating structures and biological functions of carbohydrates. Suzuki K, Tobe A, Adachi S, Daikoku S, Hasegawa Y, Shioiri Y, Kobayashi M, Kanie O.
Org Biomol Chem. 2009 Nov 21;7(22):4726-33. doi: 10.1039/b909556j. IF 3.9 - Energy-Resolved Structural Details Obtained from Gangliosides. Shioiri Y, Kurimoto A, Ako T, Daikoku S, Ohtake A, Ishida H, Kiso M, Suzuki K, Kanie O.
Anal Chem. 2009 Jan 1;81(1):139-45. doi: 10.1021/ac801611z. IF 8.0 - Fluorescence-monitored zero dead-volume nanoLC-microESI-QIT-TOF MS for analysis of fluorescently tagged glycosphingolipids. Daikoku S, Ono Y, Ohtake A, Hasegawa Y, Fukusaki E, Suzuki K, Ito Y, Goto S, Kanie O.
Analyst. 2011 Mar 7;136(5):1046-50. doi: 10.1039/c0an00715c. IF 5.2 - Synthesis of a fluorescently tagged sialic acid analogue useful for live-cell imaging. Suzuki K, Ohtake A, Ito Y, Kanie O.
Chem Commun (Camb). 2012 Oct 9;48(78):9744-6. doi: 10.1039/c2cc34605b. IF 6.1 - Analysis of the Cellular Dynamics of Fluorescently Tagged Glycosphingolipids by Using a Nanoliquid Chromatography-Tandem Mass Spectrometry Platform. Ohtake A, Daikoku S, Suzuki K, Ito Y, Kanie O.
Anal Chem. 2013 Sep 17;85(18):8475-82. doi: 10.1021/ac401632t. IF 8.0 - Syntheses of lactosyl ceramide analogues carrying novel bifunctional BODIPY dyes directed towards the differential analysis of multiplexed glycosphingolipids by MS/MS using iTRAQ. Son SH, Daikoku S, Ohtake A, Suzuki K, Kabayama K, Ito Y, Kanie O.
Chem Commun (Camb). 2014 Mar 21;50(23):3010-3. doi: 10.1039/c4cc00112e. IF 6.1 - Synthetic study of 3-fluorinated sialic acid derivatives. Suzuki K, Daikoku S, Son SH, Ito Y, Kanie O.
Carbohydr Res. 2015 Apr 10;406:1-9. doi: 10.1016/j.carres.2014.12.010. IF 3.0
- The relationship between glycan structures and expression levels of an endoplasmic reticulum-resident glycoprotein, UDP-glucose: Glycoprotein glucosyltransferase 1. Daikoku S, Seko A, Son SH, Suzuki K, Ito Y, Kanie O.
Biochem Biophys Res Commun. 2015 Jun 19;462(1):58-63. doi: 10.1016/j.bbrc.2015.04.105. IF 3.3 - Evaluation of reversed-phase nano liquid chromatography conditions by using reversed-phase thin layer chromatography based on Hansen solubility parameters for the analysis of amphiphilic glycosylsphingolipid transformations. Kanie Y, Taniuchi M, Kanie O.
J Chromatogr A. 2018 Jan 26;1534:123-129. doi: 10.1016/j.chroma.2017.12.058. IF 4.6
The program now terminates.