First achievement of continuous sequential enzymatic reactions based on micro-fluidic system
Very little is known about the regulation mechanism of the synthesis of oligosaccharide at Golgi that remains to be solved. We aim to reveal the mechanism by recreating the system as a microfluidic chip.
The glycosyltransferases were immobilized on beads which were introduced into a microchannel created in a glass plate. We also developed a system enabling on-line analysis of the reactions taking place in the microchip connecting the chip through micro LC to ESI-QIT-MS.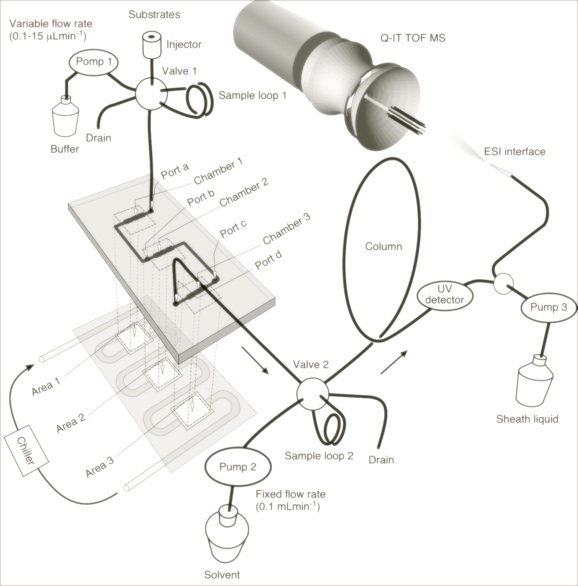
 We demonstrated sequential enzymatic reactions using a microfluidic system for the first time. As a result of three sequential reactions, a tetrasaccharide, beta-GlcA-(1-3)-beta-Gal-(1-3)-beta-Gal-(1-4)-beta-Xyl-(1-PNP was synthesized. As for the first reaction with one of the glycosyltransfereases, GalT-I, about 97% conversion was accomplished.
We demonstrated sequential enzymatic reactions using a microfluidic system for the first time. As a result of three sequential reactions, a tetrasaccharide, beta-GlcA-(1-3)-beta-Gal-(1-3)-beta-Gal-(1-4)-beta-Xyl-(1-PNP was synthesized. As for the first reaction with one of the glycosyltransfereases, GalT-I, about 97% conversion was accomplished.

Reference
Microfluidic device enabling synthesis of a tetrasaccharide aiming at “Golgi Simulator”. Ono, Y. Daikoku, S. Hasegawa, Y. Sato, T. Kobayashi, M. Suzuki, K. Kanie, O. Molecular Imaging for Integrated Medical Therapy and Drug Development, Springer, Eds. Tamaki, N. Kuge, Y., 2009, pp288-293.


