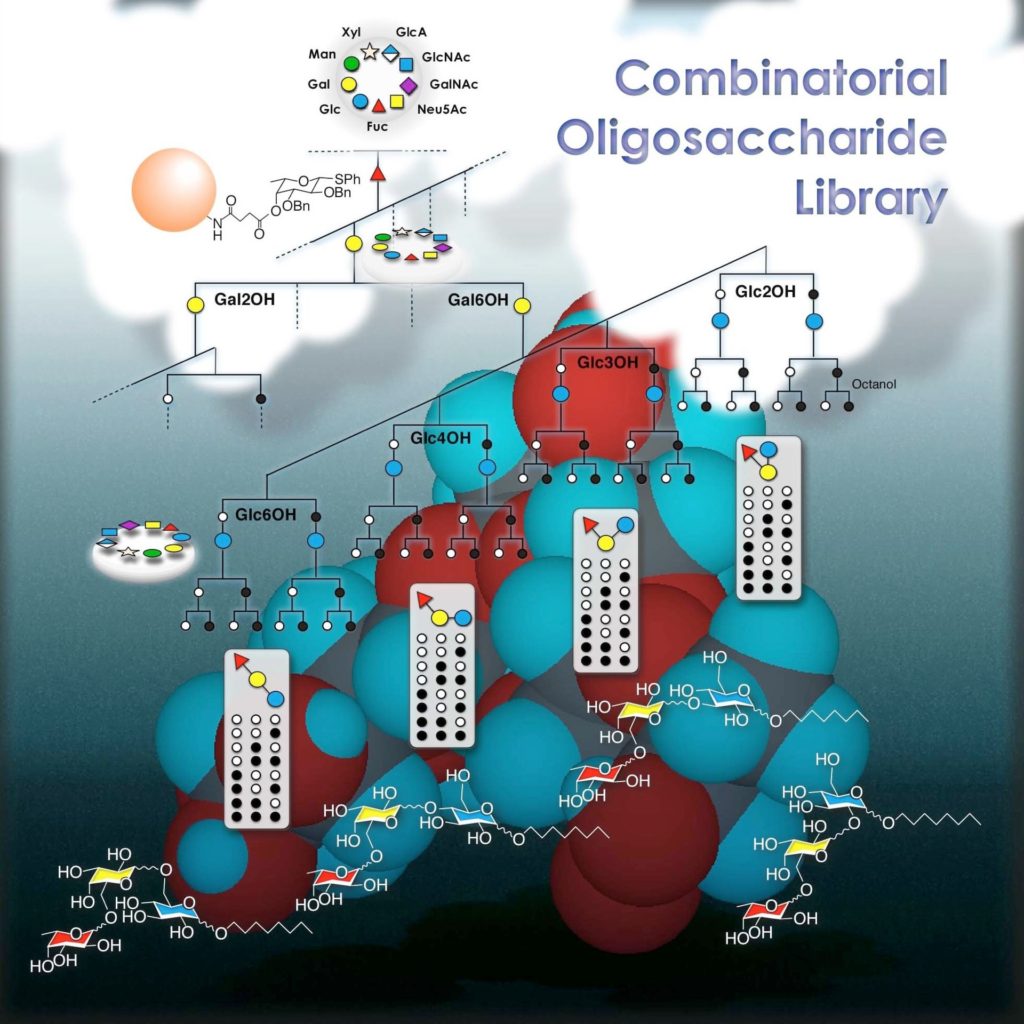
Our philosophy behind it to accomplish combinatorial oligosaccharide library
Generation of a combinatorial oligosaccharide library is one of most challenging subjects in the carbohydrate chemistry. The difficulty comes from, at first, preparation of all the necessary synthetic units required for the library synthesis. Since synthesis of individual unit requires multiple protecting group manipulation steps, preparation of all the units is just time consuming. These units have to be synthesized taking considerations for the expecting glycosylation reactions. Particularly protecting group at 2-position is important in order to control stereochemistry of forming glycoside.
Another issue is the reaction conditions to be applied. Individual molecules react under specific conditions, therefore, we often spend considerable time for optimization. However, the type of optimization process cannot be applied in the combinatorial chemistry, because the this chemistry is to speedup the synthesis for high-throughput discovery process of pharmaceuticals for example. When one achieves optimization of all the reactions, one do not have to do the chemistry any more.
We addressed above two independent issues to generate oligosaccharide combinatorial chemistry. Firstly, we proposed stereo-non-selective glycosylation that utilizes non-participating protecting group such as benzyloxy group and azide group at C-2 not because to take advantage of anomeric effect but to partially take advantage of nitril-effect to randomize stereochemical outcome. Secondly, we introduced a concept of temperature increment rate. This makes individual condition optimization unnecessary. We examined various temperature increment rate to minimize competing elimination product formation, and found out that rate should be less than 3 ˚C/h. Applying these criteria enabled us to create some series of combinatorial oligosaccharide libraries. After deprotection sequence, the anomeric mixture was purified by HPLC.
In this trend of research, we also reveled that mechanical mixing is not required for the solid-phase synthesis. Chemical reactions take inside pores of PEG-grafted polystyrene beads are controlled by the chemical diffusion. Externally applied mechanical force such as vortex mixing is not effective, although it may affect a little through supplying heat that is generated by the friction between beads. We showed that the use of minimum amount of solvent just enough to swell the beads enhances the reaction rate giving much higher yield.
Suzuki, K. et al. J. Carbohydr. Chem, 2005, 24, 219-236.
Kanie, O. et al. Angew. Chem. Int. Ed., 2006, 45, 3851-3854.
Ohtsuka, I. et al. Carbohydr. Res., 2006, 341, 1476-1487.
Ako, T. et al. Chem. Asian J., 2006, 1, 798-813.