Our obvious interest was to create a small molecule that mimics the action of a glycoprotein called macrophage activating factor (MAF). Activation of macrophage leads to anticancer activity due to the cytotoxic immune cells. MAF carries GalNAc at Thr, and the monosaccharide has been identified as an essential element for the activity, however, the increased level of a hydrolase that destroys the glycosidic bond increases in the cancer patients. For this reason MAF itself has an issue to be used as an anticancer drug.
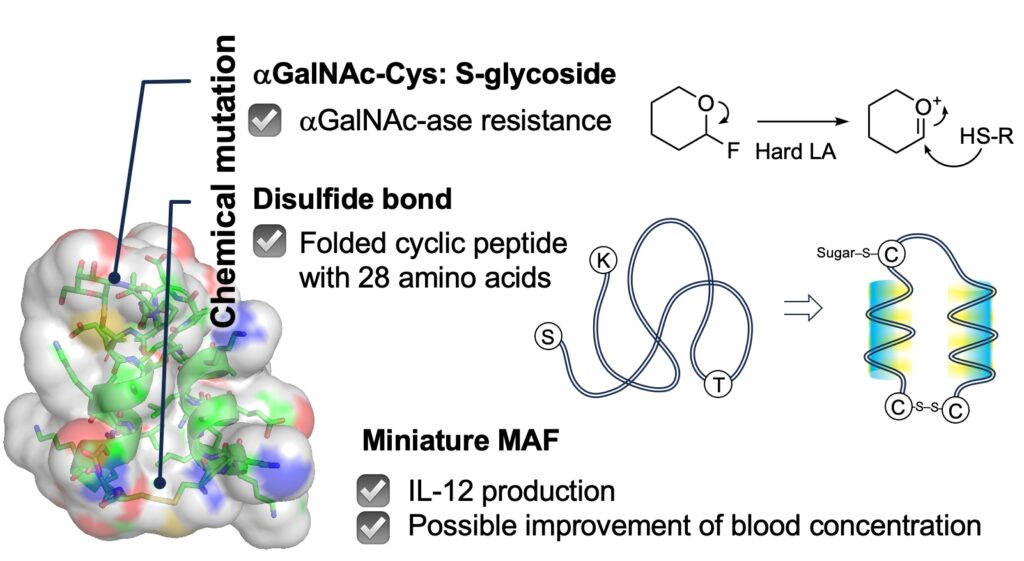
Close investigation of crystal structures of the protein made us create a miniature MAF. The requirements for the designed molecule is as follows.
(1) The designed molecule can easily be synthesized based on solid-phase peptide synthesis.
(2) The peptide sequence is closely related to the partial structure of the original MAF.
(3) The molecule has to resist against GalNAs-ase.
(4) The molecule has a fold structure resembling the active domain of MAF.
Beside the obvious target molecule, we were concerned the current problem in biological medical products. Some of such pharmaceutical drugs are glycoproteins, however, glycan synthesis cannot be controlled. Current trend of research is to replace the glycans formed by the cultured cells by chemoenzymatically synthesized pure form of glycans as needed. It seems a fine strategy for the next generation drugs, but the pure glycans are prone to the hydrolases present in the bloodstream, thus resulting in a glycoform consisting of a mixture of partially degraded shorter glycans. We need some ways to circumvent the problem that is the introduction of hydrolase-resistant glycan structures. Such introduction can be restricted at the non-reducing end terminus carbohydrates. Hydrolases generally act on the terminal sugar, so that introduction of a hydrolase-resistant carbohydrate protects the entire glycan structure attached to a protein.
We achieved the synthesis of a small glycopeptide with 28 amino acids only where GalNAc is linked to Cys replacing the original Thr for stability issue and N- and C-terminal amino acids Lys and Ser of the peptide is replaced with two Cys for disulfide bond that makes the small peptide fell into a desired helical bundle structure.
Kotaro Kanzaki, Yuma Nakagomi, Yuri Asami, Haruki Honma, Yoshitaka Yokoyama, Honoka Seki, Tomoka Imamine, Miho Nanaumi, Toshiki Izawa, Sayako Misawa, Michio Iwaoka, Naoya Kojima, Hidekazu Katayama, Yoshimi Kanie, and Osamu Kanie